What is Crosslinking?
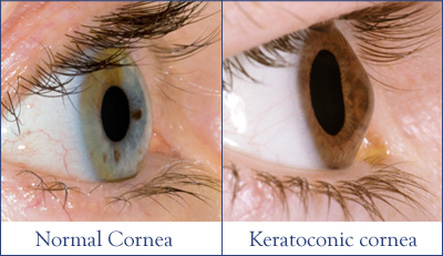
Corneal Crosslinking is a gentle, safe and effective treatment for Keratoconus, Pellucid Marginal
Degeneration (a form of Keratoconus) and post-LASIK ectasia. These are all progressive thinning
disorders of the cornea – the clear outer surface of the eye – which can lead to vision loss and the need for corneal transplantation.
Corneal Crosslinking has been utilized worldwide since the 1990’s but has only recently come to the U.S. The U.S. Food and Drug Administration have given approval to a form of Crosslinking different than that offered in this clinical trial. That form involves the removal of the surface of the eye prior to the application of the riboflavin. The exposure time to the U.V. light source is 30 minutes long and healing times can be extended and fairly uncomfortable.
Corneal Crosslinking offers patients with keratoconus a minimally invasive procedure to help correct their vision problem.
FDA-Approved Clinical Trial
In our clinical trial, we are utilizing a different form of Riboflavin (B2) that does not necessitate the removal of the surface of the cornea, as well as a different U.V. light source that requires less exposure time. These lead to much shorter and more comfortable healing times. This treatment has long been considered the standard of care in Europe and elsewhere in North America.
Who is a good candidate for Crosslinking?
Ideal candidates for Crosslinking are those identified early in their lives with keratoconus. We are able to treat children as young as 15 years old and adults up to age 50. Children with a family history of keratoconus should be screened as early as possible.
How do I schedule a screening or a procedure?
When you are ready to learn more, or take that next step in stabilizing your vision, call us to schedule a consultation!
